Greetings! Researchers from the Office of Science and Engineering Laboratories, CDRH at the US FDA, alongside academic, clinical and industry colleagues, are collecting pathologist annotations of stromal tumor infiltrating lymphocytes (sTILs) as data for AI/ML algorithm validation.
Currently, due to regulatory expectations, we are requiring participants to be board-certified U.S. pathologists or international equivalent. This requirement may be relaxed in the future depending on regulatory feedback.
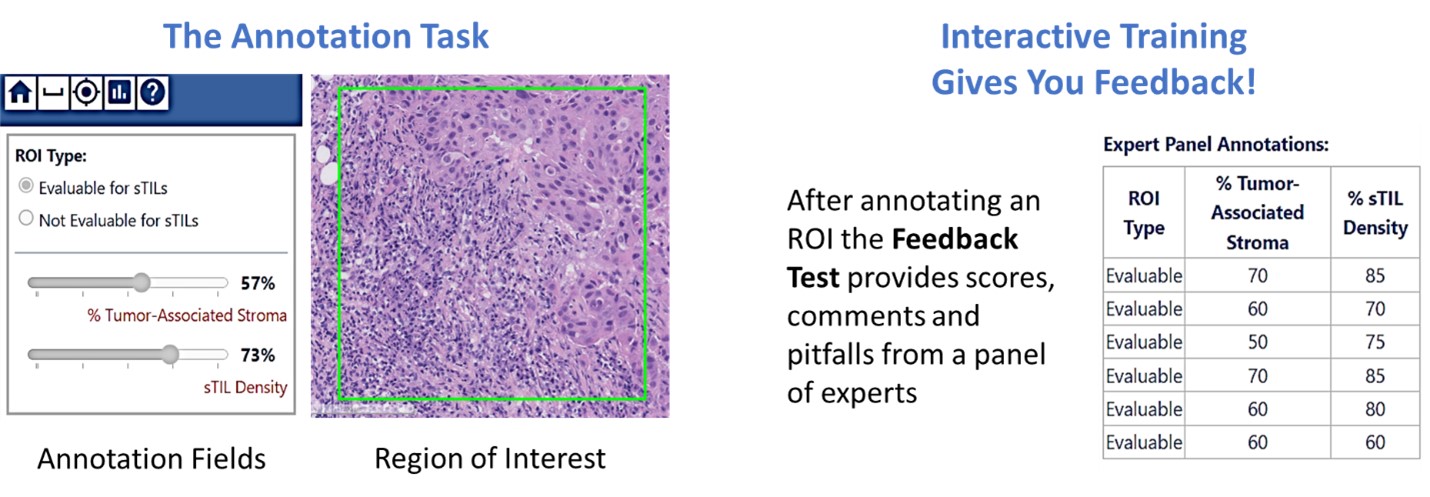
Volunteer pathologists will receive training in the task through a continuing medical education course (3.00 CME credits) and interactive training on our digital data-collection platform. After training, participants will be assigned pivotal study collections (30-60 min per collection) according to their interest and availability. Through your involvement, you will be generating the reference standard for algorithm validation ensuring high quality commercial products with a faster FDA-pipeline to approval.
Questions? Email the project management team